at Tokyo Institute of Technology, National Institute of Materials, and Chiba University
<1988 - 2004>
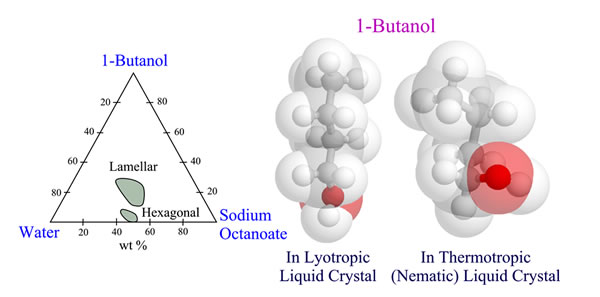
We carried out conformational analysis of chain molecules incorporated in liquid crystalline phases (LCs). We treated n-alkanes, ethers, alcohols, and oligomeric model compounds of polymer liquid crystals dissolved in thermotropic LCs and, in addition, amphiphilic molecules (alcohols and carboxylates) forming lyotropic LCs. This study aimed to correlate the molecular structures with what we call the liquid crystallinity, i.e., the capability to harmonize with LC phases. Our techniques include deuterium NMR, X-ray diffraction, thermal analysis, molecular orbital calculations, and the rotational isomeric state (RIS) scheme.
Chain Molecules Incorporated in Nematic Liquid Crystalline FieldsPublication List
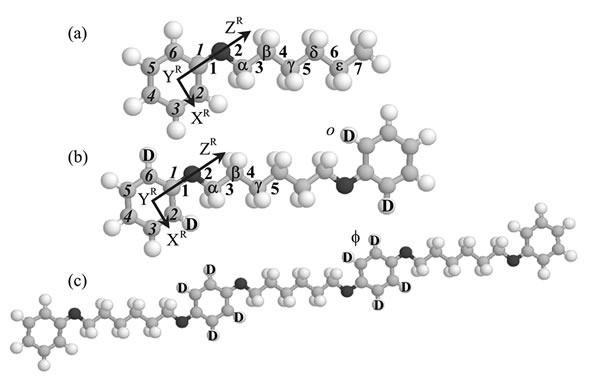
We developed a simulation scheme based on the RIS scheme and the maximum entropy (MaxEnt) method to analyze deuterium NMR quadrupolar splittings and dipolar couplings observed from deuteriated solutes in LC solvents. The RIS & MaxEnt method was applied to various nematic systems to derive bond conformations and orientational order parameters of chain molecules such as n-alkanes, ethers, and alcohols dissolved in nematic LCs.
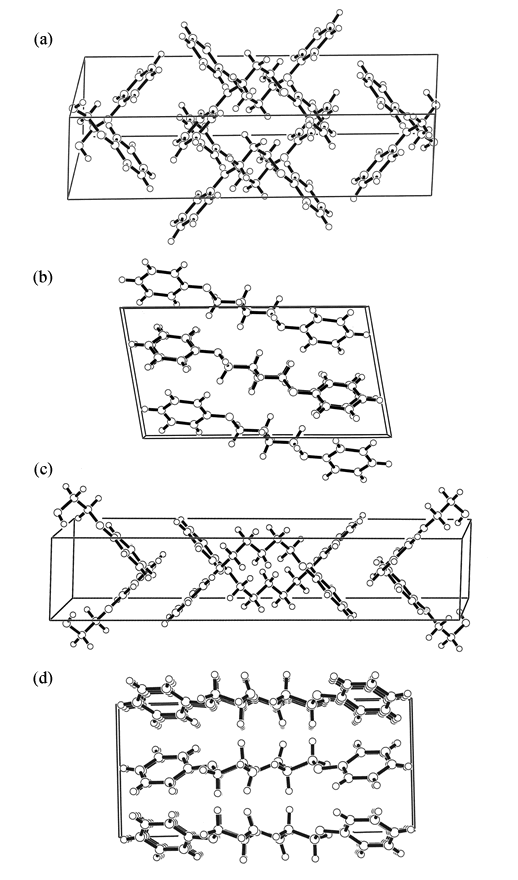
We also treated oligomeric model compounds of polymer LCs. These compounds were dissolved in nematic LCs such as 4'-methoxybenzylidene-4-n-butylaniline (MBBA) and p-azoxyanisole (PAA), the phase transitional behaviors were investigated, and the deuterium NMR data were analyzed by the RIS & MaxEnt method. Their crystal structures were also determined by single crystal X-ray diffraction. The liquid crystallinity of the model compounds was discussed in terms of conformational characteristics, and the origin of the so-called odd-even oscillation characteristic of LC molecules was elucidated.
Aggregation Structures of Amphiphilic MoleculesPublication List
We found the following “peculiar” conformational behaviors of amphiphilic molecules in LC phases. Nematic LC fields render n-alkanes and ethers somewhat rigid and extended. However, even in the nematic phase, alcohol molecules tend to adopt gauche conformations in C−C bonds near the hydroxyl terminal, thus being rather spherical. When carboxylate and alcohol molecules are incorporated in lyotropic LC phases (hexagonal, reverse hexagonal, and lamellar aggregates), the longer amphiphilic molecule will prefer the “peculiar” conformations so as to fill both interface and inner space effectively and stabilize the aggregate structures.